Pfizer, Moderna Jump After FDA Clears Covid Booster Shot For Adults
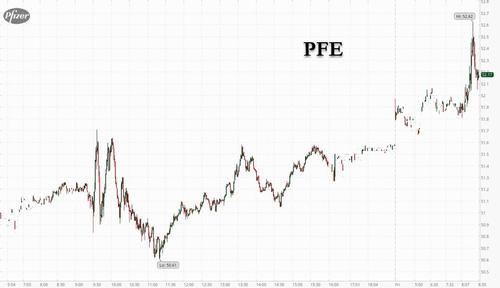
Update (830am ET): Pfizer has joined Modern in trading sharply higher – and hitting new record highs – when moments after the Moderna announcement, Pfizer and BioNTech also said the U.S. Food and Drug Administration has expanded the emergency use authorization of a booster dose of the Pfizer-BioNTech COVID-19 vaccine to include individuals 18 years of age and older. The booster dose is to be administered at least six months after completion of the primary series, and is the same dosage strength as the doses in the primary series.
* * *
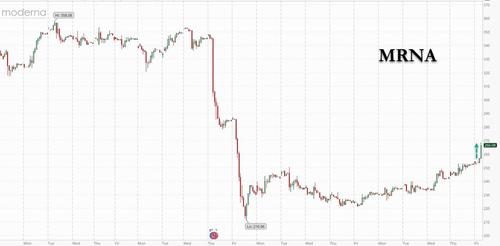
Outright bans across numerous (mostly Nordic) countries due to widespread reports it is causing myocarditis in children and adults? No problem: moments ago the FDA cleared Moderna’s Covid-19 booster shot for all Americans 18 and older, making millions more people eligible for extra protection as concern about a potential winter wave of infections grows. As to whether those millions will take the booster shot knowing that the “vaccine” doesn’t actually prevent them from catching covid, and at best mitigates the severity of the disease even as it creates risks of other, just as unpleasant side-effects, is unclear.
The U.S. Food and Drug Administration said adults who received their second dose of the company’s shot at least six months ago are now eligible to receive a third, according to a statement Friday from Moderna. The booster from Pfizer Inc. and BioNTech SE is expected to gain a similar clearance on Friday.
The FDA based the EUA on scientific evidence shared by the company including a data analysis from the Phase 2 clinical study of mRNA-1273, which was amended to offer a booster dose of mRNA-1273 at the 50 µg dose level to interested participants 6-8 months following their second dose (n=344)
A panel of experts who advise the Centers for Disease Control and Prevention on vaccine policies are expected to review the evidence and make further guidance for administering booster shots at a meeting on Friday afternoon; there will be no surprises as the CDC will side with the FDA.
Booster shots of mRNA vaccines – also called “gene therapy” by those who then see their twitter account be banned in perpetuity – have been available for people 65 and over, as well as others who could be at higher risk of severe disease, since mid-September. Under federal guidelines, recipients can receive any of the three authorized shots as a booster, regardless of the brand of their initial vaccine.
More than a third of fully vaccinated seniors had received a booster as of Thursday, according to the Centers for Disease Control and Prevention, and 17% of fully vaccinated adults overall have had an additional shot.
Worry that the effectiveness of initial inoculations could fade as the weather cools and more people socialize indoors has driven the most recent push for wider use of boosters. Data presented to U.S. drug regulators by Pfizer, Moderna and Johnson & Johnson suggested all three of the currently authorized vaccines in the U.S. have seen their efficacy diminish.
Pfizer asked the FDA to expand access to its booster to all adults earlier this month. The company and partner BioNTech SE said data from a large clinical trial demonstrated a relative vaccine efficacy of 95% for booster recipients when compared with those who didn’t get a booster.
Moderna shares surged as much as 6.8% to $268.70 in premarket trading
Tyler Durden
Fri, 11/19/2021 – 08:30
Zero Hedge’s mission is to widen the scope of financial, economic and political information available to the professional investing public, to skeptically examine and, where necessary, attack the flaccid institution that financial journalism has become, to liberate oppressed knowledge, to provide analysis uninhibited by political constraint and to facilitate information’s unending quest for freedom. Visit https://www.zerohedge.com